Abstract
Background: Stroke is a devastating complication of sickle cell anemia (SCA). The STOP (Stroke Prevention Trial in Sickle Cell Anemia) protocol was adopted in 2014 by the National Heart Lung and Blood Institute (NHLBI) guidelines for stroke-risk screening using transcranial Doppler ultrasound (TCD) and prevention with chronic red cell transfusion therapy (CRCT). These guidelines were upheld in the recent American Society of Hematology guidelines. Despite strong evidence supporting TCD screening (and CRCT initiation) in stroke prevention, wide scale implementation has not been achieved 4. DISPLACE (Dissemination and Implementation Looking at the Care Environment) is a multicenter, NHLBI-funded study conducted to identify barriers to TCD implementation and test novel methods for improving outcomes. DISPLACE is a 3-part study: 1) Retrospective assessment of current practice; 2) Assessment of barriers and facilitators; and 3) Implementation trial to improve TCD screening. This report focuses on Part 3.
Methods: DISPLACE is a 28-center consortium across the US representing a range of: region (urban vs rural), size, and institution type (academic vs community). During Part 1, sites performed a rigorous retrospective chart review of children with SCA aged 2-16 years from 2012-2016 to collect baseline TCD rates. Part 2 included a rigorous mixed methods of the barriers and facilitators assessment to TCD screening was performed that resulted in 3 main interventions: 1.Re-branding TCD as Sickle Stroke Screen, a meaningful name selected by patients/families with SCD; 2. A Single Coordinator (SC) for each site's TCD screening, scheduling, and follow-up; 3. A newly designed application, Provider Minder (trademarked; PM).
PMwas created to remind providers of patient appointments (versus reminders only sent to patients/families). Data entry in PM included birth date (to calculate age), city/state of residence, distance from SCD clinic and insurance. Date of last TCD was recorded to calculate date of next screen. When the TCD was due, the system alerted the provider to enter appointment completion or reason(s) why it was missed. TCD results were entered for PM to prompt for the next TCD date in a 10-14 month window (normal screens), 2-6 weeks (conditional screens), and intervention plan (abnormal screens): repeat scan in <2 weeks or other. If the provider failed to enter a patient's result, PM alerted the provider q3 days until the TCD was recorded as completed/rescheduled.
Part 3 included 16 sites who successfully completed data entry in Part 1 and had the lowest TCD implementation rates. Part 3 inclusion criteria were the same as Part 1 except to specifically select children with SCA now aged 2-7 (the highest risk group to have an abnormal TCD in Part 1). All 28 sites received re-branding educational material (also disseminated to non-study sites). Cluster randomization was used to allocate sites to PM (8 sites) or PM + SC (8 sites). The primary outcome is which intervention results in the best TCD rates. Secondary outcomes include Part 3 sites’ improvement in TCD rates (compared to historical rates) and impact of the educational/rebranding of TCD to "Sickle Stroke Screen” in sites (4) that did not participate in Part 3. Stroke status, presence of CRCT after an abnormal TCD, missed TCD appointments, and medical decisions following TCD were also recorded.
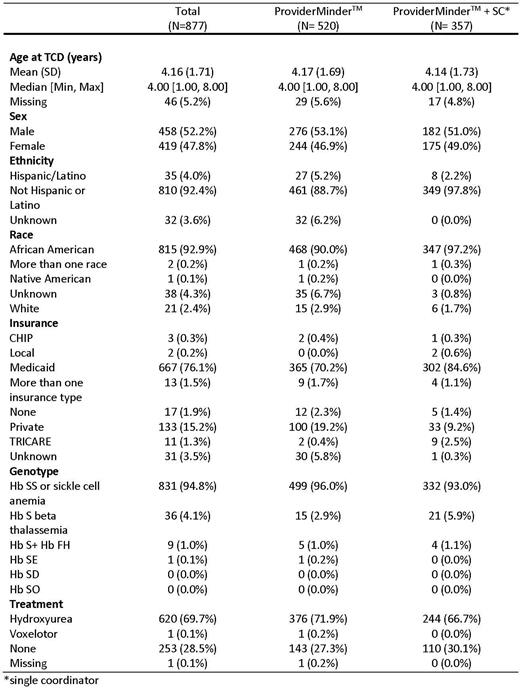
Results: Part 1 included 28 centers with 5223 individuals with SCA in the database. Of the 16 Part 3 sites, three could not implement the interventions due to a simultaneous change in hospital administration and site PI (1), inability to use PM due to institutional firewall (1) and inability to implement due to site specific issues (1). These 3 sites remained in the study as "control sites” and a fourth control site was added. Thus, 13 sites implemented the interventions with 877 patients; 520 were in sites using PM and 357 were in sites using PM+SC. Demographic data for the 877 children with SCA are in Table 1. The study ended on May 31, 2022. Calculations of TCD rates at all sites are in process and will be presented.
Conclusions: Despite the benefit of TCD screening in children with SCA, guideline-based implementation remains poor. The range of barriers to screening has been previously reported by the DISPLACE study team and were used to design the study interventions. Results from this study will guide optimization of the intervention protocol proven to enhance TCD implementation.
Disclosures
Kanter:GLG: Consultancy; Fulcrum Tx: Consultancy; Forma: Consultancy, Membership on an entity's Board of Directors or advisory committees; University of Alabama Birmingham: Current Employment; Novartis: Consultancy, Honoraria; Ecor1: Honoraria; Guidepoint Global: Honoraria; Beam: Honoraria; Graphite Bio: Consultancy; ORIC: Consultancy; Bausch: Consultancy, Honoraria. Adams:Global Blood Therapeutics: Honoraria; MUSC: Current Employment; Zeriscope Inc: Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal